
Starch Gelatinization
What is Starch Gelatinization?
Starch gelatinization is the irreversible loss of the molecular order of starch granules (crystallinity). It is considered a glass transition from an ordered initial state to a disordered final state, usually resembling a “melting” process, that requires water and heat.1,2
In the cooking or baking process, it’s the stage where starch granules swell and absorb water, becoming functional.
How does it work?
Native starch is partially crystalline and highly organized, a result of interactions between amylose and amylopectin fractions which also reduce its water solubility. When dispersed in excess water at room temperature, starch granules only take up about 30–40% of their dry weight as moisture, causing them to swell slightly and settle to the bottom. However, this process can be reversed.
Starch in hot water
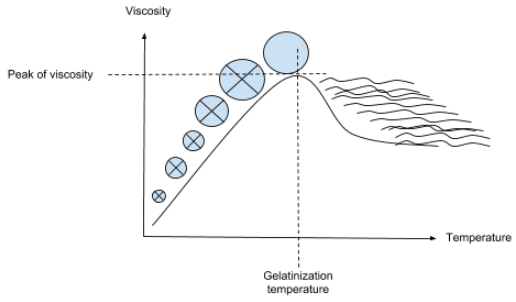
During heating and in the presence of excess water, starch granules initially imbibe (bind) water causing them to gradually swell and form a viscous slurry. As heating continues and temperature increases, the granules start losing their crystallinity becoming amorphous as evidenced in the disappearance of the Maltese cross (birefringence) observed via light microscopy.3
Subsequent heating causes the granules size to increase until they can no longer absorb more water and burst. Rheologically, this is accompanied by maximum viscosity build up followed by a drop to a plateau. As molecules making up the granule start to leach out from the swollen granules and disperse/solubilize in the aqueous medium, yield a gel or paste whose properties depend on the concentration and type of starch in the slurry.1,2
The amylose and amylopectin fractions start to solubilize at 158°F (70°C) and 194°F (90°C), respectively. These fractions become loose and eventually become more reactive and prone to enzyme attack (especially amylases).1,2 The following schematic representation shows how the mode of starch granules swelling and loss of birefringence.
Application
During baking, gelatinized starch absorbs free water in the dough. As gas bubbles in the dough expand and eventually burst to form an air-continuous or porous structure. The starch gel/coagulated protein matrix surrounding these bubbles increase in viscosity to form a firm structure, essential for setting bread structure and crumb texture.
Extent of starch gelatinization varies with:
- Temperature
- Heating rate and extent of heating
- Available water (aw)
- pH
- Type of starch (source)
Impact of water activity on gelatinization
The presence of dissolved solids and low molecular weight compounds such as salts, sugars, amino acids and alcohols (e.g. polyols and glycerol) lowers the amount of free or unbound water, thus necessitating higher temperatures for the starch to gelatinize. This is the reason why bakery formulas rich in sugar and fat and low in water, such as pie crusts and cookies, never attain complete starch gelatinization.
Such formulations delay the set of crumb (firming) in doughs and batters during baking. Therefore, for optimum expansion volume build up during oven spring, the dough/batter needs to remain somewhat flexible or viscous to allow leavening gases to expand.
Gelatinization temperature of starches from select plants
The following table summarizes the gelatinization temperature of various starch sources:4,5
| Source | Gelatinization temperature |
| Wheat | 124–140°F (51–60°C) |
| Barley | 124–140°F (51–60°C) |
| Corn | 144–162°F (62–72°C) |
| Triticale | 131–144°F (55–62°C) |
| Rice | 154–172°F (68–78°C) |
| Rye | 124–140°F (51–60°C) |
| Sorghum | 154–172°F (68–78°C) |
| Potato | 140–149°F (60–65°C) |
| Tapioca | 153–158°F (67–70°C) |
Methods used to study starch gelatinization
This phenomenon can be studied using techniques such as:4
- Optical microscopy
- Amylography
- Rapid visco-analysis (RVA)
- Differential scanning calorimetry
- Time-resolved X-ray diffraction analysis
References
- BeMiller, J.N. “Starches: Molecular and Granular Structures and Properties.” Carbohydrate Chemistry for Food Scientists, 3rd edition, AACCI and Elsevier Inc., 2019, pp. 159–182.
- Yongfeng, A., and Jay-lin, J. “Understanding Starch Structure and Functionality.” Starch in Food Structure, Function and Applications, 2nd edition, Woodhead Publishing, Elsevier Inc., 2018, pp. 151–169.
- Finnie, S., and Atwell, W.A. “Composition of Commercial Flour.” Wheat Flour Handbook, 2nd edition, Cereals & Grains Associations, AACC International, Inc., 2016, pp. 35–41.
- Delcour, J.A., Hoseney, R.C. “Starch.” Principles of Cereal Science and Technology, 3rd edition, Cereals & Grains Associations, AACC International, Inc., 2010, pp. 33–45.
- BeMiller, J., and Whistler, R. “Tapioca/Cassava Starch: Production and Use.” Starch: Chemistry and Technology, 3rd edition, Academic Press, Elsevier Inc., 2009, p. 550.

