
Baker’s Ammonia
Also known as Ammonium Carbonate, Hartshorn Salt or Baker’s Salt
What is Baker’s Ammonia?
Baker’s ammonia is an alkaline chemical leavening agent used in the baking industry. It is an alternative to the commonly used baking soda and baking powder. Typically, Baker’s ammonia is associated with unique crispness and light texture in baked goods.
Chemical Structure
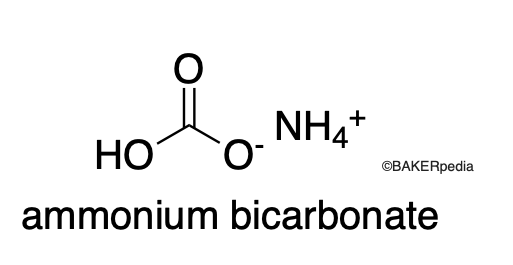
Origin
Baker’s ammonia was introduced to home bakers in the 1830s. It was originally made by grinding up the horns of deer and was typically used in Greek, German and Scadinavian cuisines.
Today, it is obtained through the chemical reaction of ammonia and carbon dioxide.
Function
When exposed to high temperatures above 40°C (104 °F), Baker’s ammonia decomposes into ammonia, water and carbon dioxide. All three of them contribute to the leavening of baked goods.¹
Critical functions of Baker’s ammonia contributes in baked goods include:¹
- Leavening: gases are formed from the breakdown of baking soda, and later expand during the baking process.
- Tenderizing: the produced gases expand the cell walls of baked goods, making them more tender.
- pH adjustment: unlike baking soda, it has minimal effect on the pH of baked goods.
- Provides a finer crumb
- Modifies flavor and taste: in cake batters, it can generate undesirable flavor/aroma as well as saltiness.
- Dough strengthener
Commercial production
Baker’s ammonia is commercially produced through the following process: 4
- Synthesis: carbon dioxide (gas) is mixed in countercurrent with an aqueous solution of ammonia. It is typically carried out in a packed tower or absorption column.
- Crystallization: the resulting saturated ammonium bicarbonate solution is allowed to crystallize.
- Filtration: crystals are filtered or centrifuged to separate the product from the waste stream.
- Washing
- Dryng: obtained crystals are air dried at 50 °C (122 ° F).
An alternative process involves a reaction of ammonium chloride or ammonium sulfate and chalk in iron retorts followed by sublimation.
Application
Baker’s ammonia is typically used in the manufacture of low moisture (<5%) baked goods like German cookies (Basler Leckerli and Spekulatius), cream puffs and crackers. It is not recommended for large scale production, and high or intermediate moisture products like muffins, biscuits, cakes, and soft cookies.
Commonly observed effects of baker’s ammonia in baked goods are:
- Quick reaction in the presence of water and heat
- The reaction rate may increase in the presence of acids.
- Increases uniformity and spread in cookies.
- Increases browning.
- Increases crispiness and porosity of the crumb.
- It may impart an undesirable ammonia-like taste and odour, if not fully evaporated.
- It is not very active at room temperature.
- It is considered a fast-acting leavening agent.
- Eggs content can be reduced when using baker’s ammonia.
- 1 part of Baker’s ammonia can be substituted with 1 part of baking powder. In certain formulations, it is replaced by 1 part baking powder plus 1 part baking soda.
Regulations
Baker’s ammonia is considered GRAS by the FDA when used within current good manufacturing practices, and it is regulated by the CFR Title 21 Part 184.4
In the EU, baker’s ammonia (E 503 ii) is regulated by the EU commission No 231/2012.5
References
- Figoni, P. How Baking Works: Exploring The Fundamentals Of Baking Science. 2nd ed., John Wiley & Sons, Inc., 2008.
- Suas, M. Advanced bread and pastry. Nelson Education, 2012.
- Hui, Y., Corke, H., De Leyn, I., Nip, W.N and Cross, N .Bakery products: science and technology. John Wiley & Sons, 2008.
- U.S. Department of Health and Human Services.” Direct Food Substances Affirmed As Generally Recognized As Safe”.Title 21 Code of Federal Regulation, Part. 184. April 2019. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1135 . Accessed 17 September 2020.
- European Commission (EC). Commission Regulation NO231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council . Official Journal of European Communities, 09 March 2012.

