Trans Fat
Also known as trans fatty acids or TFA
What is Trans Fat?
Trans fat is a type of fat/oil which contains fatty acids with at least one trans bond. Trans fats are generally formed as side-reactions during hydrogenation but can also occur naturally in certain fats from animal sources.1
Trans fat behaves like saturated fat in the body by raising low-density lipoprotein (LDL) or “bad” cholesterol which can increase the risk of coronary heart diseases.2,3
Origin
In the trans configuration, the hydrogen atoms on the double bond in a fatty acid chain are opposite each other. As a result the chain is nearly straight (with a slight kink at the double bond), as shown in the figure below. The cis-isomers prevail in all the food fats and oils, although small amounts of trans-isomers occur in fats from ruminants.3
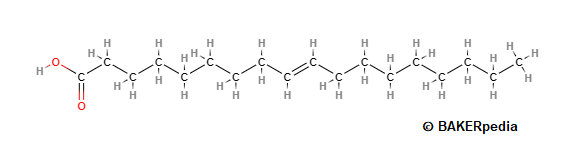
Elaidic acid (EA, C18:1 trans), which is the trans form of oleic acid (OA, C18:1 cis), is the principal TFA found in partially hydrogenated vegetable oils and margarines.
Trans fats consumed in foods have biological or technological origin where a significant amount of them comes from the so-called “unseen fat.” This is used as the raw material for the manufacture of processed foods.
Grazing animal fats found naturally in meat, milk and dairy products can contain trans fats, although in lower amounts than those found in processed vegetable oils and animal fats.
Nutrition
High consumption of trans fat is a risk factor for cardiovascular health. It increases the risk of bleeding and thrombosis because these fats are atherogenic, that is, they stick to the walls of arteries, thickening them and making them rigid. WHO and FAO recommend that consumption of trans fatty acids be less than 1% of daily calories.3
The concentration of trans double bonds in a fat or oil sample can be measured by infrared spectrophotometry. The sharp absorption peak at 968 cm-1 is used to quantify the amount of trans double bonds in the fat sample suspended in a solvent such as carbon disulfide.1
Commercial production
The formation of trans fats is a result of transforming liquid oils into solid fats, such as shortening or stick margarine. During this process, called hydrogenation, hydrogen is added to vegetable oil to increase the shelf life and stabilize the flavor of processed foods. The result of the process is trans fats.
Trans fat is a component of partially hydrogenated oils (PHOs). The partial hydrogenation process introduces hydrogen to the double bonds of vegetable oils and converts some unsaturated fatty acids to saturated fatty acids. This process solidifies the oil, increases its resistance to rancidity, and changes its function. PHOs impart a very smooth, creamy, desirable texture to food products that require tenderization and moisture.
Margarine, industrial fat, oil, household and industrial frying oil, confectionery and bakery products, popcorn, pasta, sweets, chocolates, fast food and a variety of snacks (snacks) such as chips, fried bananas, etc.often contain some trans fats.
Regulation
The U.S. Food and Drug Administration (FDA) recommends choosing foods low in saturated fat and cholesterol by following this general rule of thumb: 5% Daily Value or less is low, and 20% Daily Value or more is high. The EU has set 2% as the legal limit of trans fats in processed foods.
Trans fat has no Daily Value, so it is recommended to keep the consumption of foods containing trans fat as low as possible.
References
- Stauffer, C.E. Fats and Oils, American Association of Cereal Chemists, Inc., 1996.
- U.S. Food and Drug Administration (FDA), Trans Fat, https://www.fda.gov/food/food-additives-petitions/trans-fat, Accessed 30 January 2021.
- World Health Organization, Nutrition: Trans fat, https://www.who.int/news-room/q-a-detail/nutrition-trans-fat, Accessed 30 January 2021.


