
Chelating Agent
Also known as sequestrant, chelants, or metal scavengers
What are Chelating Agents?
Chelating agents are food additives that prevent oxidation and increase shelf life of baked goods. They sequester metals, preventing them from taking part in color or flavor deterioration. Chemically, chelating agents are organic compounds with a ring-like center which forms at least two bonds with the mineral ion to produce complex structures, referred to as chelates.1
Examples of include:
- Ethylenediaminetetraacetic acid (EDTA)
- Polyphosphates
- Organic acids such as citric or tartaric
Function
The word ‘chelate’ is derived from the Greek ‘chele’ which means crab’s claw. Two key characteristics are essential for chelating agents’ functionality:1
- The presence of two different binding sites for the metal it chelates.
- Ability to coordinate with the metal ion to achieve a ring formation.
Metals such as calcium, zinc, iron, copper and many others can interact with components of food systems or can act as cofactors for enzymatic activity. By binding metals, chelating agents can delay/retard these activities, thus preserving the functional and sensory properties of food products. Some chelating agents can also act as effective antioxidants.
For example, EDTA is the most universally known chelating agent. Several salts of EDTA are produced mainly calcium disodium EDTA, disodium EDTA, tetrasodium EDTA, etc.
Chemical structure of EDTA
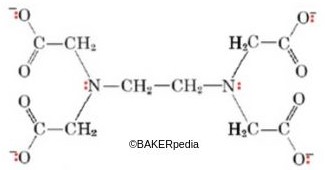 Characteristics of EDTA species:
Characteristics of EDTA species:
- Bind tightly to pro-oxidants such as iron (Fe2+) and copper (Cu+).
- Work in aqueous media but not in fats and oils
- Prevent the development of metallic taste.
- Maintain their chelating capacity in highly acidic beverages (pH 3–5), this is in contrast to most organic acids which would lose their chelating capabilities.
- Can be used in combination with the antioxidants BHT and propyl gallate
- Cost-effective
Commercial Production
EDTA is manufactured using a variation of the original method developed by Munz in 1935 in Germany. The process involves treating ethylenediamine with formaldehyde and a source of cyanide such as HCN or NaCN.The reaction yields the tetrasodium EDTA species, which is converted in a subsequent step into the acid form.3
Natural chelating agents
The synthetic origin of EDTA and its non-biodegradable nature have prompted the search for alternative clean label ingredients such as:
- Food-Grade Activated Charcoal
- Chlorella
- Glycine
However, none of these has been successfully used as a replacement for EDTA.
Application
Chelating agents are used in a wide range of food products and are FDA-approved additives in:
- Canned products
- Carbonated beverages
- Mayonnaise
- Baking mixes
- Salad dressings
- Shortening
- Potato products
- Pickled vegetables
A few common kinds in baking:
Ethylenediamine (EDTA): The most universally known chelating agent, EDTA serves as a multi-dentate molecule because it can form two different bonds.
Disodium pyrophosphate: Created by the chemical reaction of phosphoric acid and sodium carbonate, yielding sodium phosphate which must be heated further to achieve a second yield of disodium pyrophosphate. In baking, the primary function of disodium pyrophosphate is that the compound serves as a source of acid to react with baking soda resulting in leavening. During fermentation, leavening enables rise in the product due to the formation of carbon dioxide gas creating air pockets within the crumb as well as the evaporation of alcohol. A second function is increased water retention capacity within the baked item leading to increased moisture and longer shelf life.
Phosphoric Acid: Functions as a chelating agent preventing oxidation caused by metal ions.
Citric Acid: Serves the capacity to slow the rate of discoloration as well as preserve aroma. Utilized as a preservative in fruit fillings of pastries and other baked items.
FDA Regulations
Chelating agents approved by the FDA in foods include:
| Chelating agent | Regulation in 21 CFR |
| EDTA-CaNa2.2H2O | 172.120 |
| EDTA-Na2H2.2H2O | 172.135 |
| Citric acid | 184.1033 |
| Potassium citrate.2H2O | 184.1625 |
| Sodium citrate.2H2O | 184.1751 |
References
- Sharpe, P.C., D.R. Richardson, D.S. Kalinowski, and Bernhardt, P.V. Synthetic and natural products as iron chelators, Curr. Top. Med. Chem. 2011, 11, pp: 591–607.
- Rizvi, M.A., R.M. Syed, and Khan, B. Complexation effect on redox potential of iron(III)-iron(II) couple: a simple potentiometric experiment, J. Chem Ed. 2011, 88, pp: 220–222.
- Hart, J. Roger. “Ethylenediaminetetraacetic Acid and Related Chelating Agents”. Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a10_095

