
Glass Transition
What is Glass Transition?
Glass transition (Tg) is a physical property of food polymers. It is the temperature range where food polymers undergo a phase change from rigid/glassy to soft. Food polymers can be proteins, starch and non-starch polysaccharides, including cellulose, glucans and arabinoxylans.
In summary, glass transition is a physical transformation from a “glassy” state to a “rubbery” state. This means going from a lower to a higher molecular mobility and kinetic energy.1
How does it work?
Although not fully understood, glass transition plays a critical role in the processing, storage and shelf-life of cereal-based products.
There are two ways of triggering glass transitions: water and temperature. The following diagram helps visualize the glass transition of food materials such as bread, flour or cookies (naturally rich in protein and starch).1,2,3
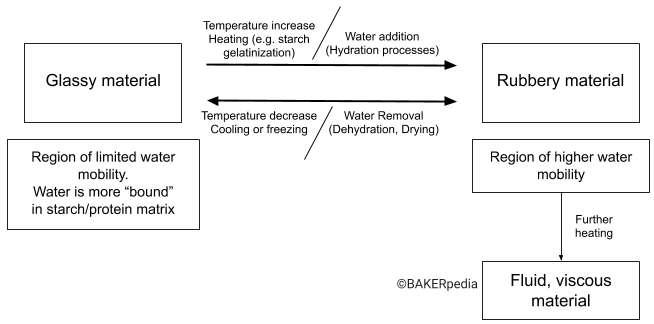
It is important to note that the words “rubbery” and “glassy” are only descriptors of the physical state of the food system rather than the texture or eating quality of the food material.
Application
In order for a material to go from a glassy to rubbery state, it must first pass through the glass transition temperature (Tg). The Tg is the temperature range when a given food material undergoes a large change in its modulus (stress force/strain ratio).2 Below its Tg, a food material is in its glassy state, and above its Tg it is in a rubbery state.
Factors that affect Tg:2,3
- Water activity (free water): The higher the water activity and/or moisture content, the lower the Tg. The presence of water depresses the Tg of food materials.
- The presence of water-binding molecules like alcohol, polyols and sugars decreases water activity and increases Tg. In the case of high-ratio cakes, sugar affects the starch gelatinization by increasing the Tg. This inhibits full gelatinization of the starch, and therefore results in a crumb that is not set. This is why high-ratio cake batters run the risk of being underbaked.
- The molecular weight of polymers affects its Tg. A high molecular weight polymer would have a higher Tg. This is why starch and gluten proteins in the dough require more heat during baking, and requires significant heat removal for proper freezing.
Glass transition mediated by water
- Sprouting or malting grain. Dry, friable and hard barley or wheat kernels are in the glassy state before they are steeped in water. During steeping, the kernels goes soft, undergoing a glass transition. This glass transition happened without a change in temperature.
- Dough mixing. During dough mixing, starch and gluten proteins interact with water molecules. Here, water acts as a plasticizer, providing molecular mobility and allowing a glass transition to occur. In this scenario, the flour (the glassy material) transforms into a dough.
Glass transition mediated by temperature
- Baking and cooling. Looking at the diagram, if starch in flour is hydrated and heated, it will undergo a glass transition known as gelatinization. The baking step is a complex process that causes both a drying (water removal) and a heating effect. At baking temperatures, a portion of the starch fully gelatinizes and proteins coagulate. This results in a flexible, resilient and porous matrix created by the starch and protein. When the starch is cooled, it will retrograde into a rigid material turning from a rubbery state to a glassy state.
- Freezing. Bread that is high in moisture and water activity is in a rubbery state at room state. When bread is frozen to extend its shelf-life, it goes from rubbery to glassy material. In this case, the temperature change is the driving force for the glass transition.
Tg measurement
Glass transition can be assessed using various techniques, the two most important ones are:
- Differential scanning calorimetry (DSC): measures the differential heat flow between a polymer sample and an inert reference at atmospheric pressure.
- Dynamic mechanical thermal analysis (DMTA): measures the polymer deformation by sinusoidally varying stress applied to the polymer. This test is a function of time, temperature and frequency around its Tg.
References
- BeMiller, J.N. “Polysaccharides: Properties.” Carbohydrate Chemistry for Food Scientists, 3rd edition, AACCI and Elsevier Inc., 2019, pp. 103–109.
- Ahmed, J., and Rahman, S. “Glass Transition in Foods.” Engineering Properties of Foods, 4th edition, CRC Press, Taylor & Francis Group, LLC, 2014, pp. 93–111.
- Delcour, J.A., Hoseney, R.C. “Glass Transition and Its Role in Cereals.” Principles of Cereal Science and Technology, 3rd edition, Cereals & Grains Associations, AACC International, Inc., 2010, pp. 89–96.

