
Cocoa Alkalization
Also known as dutched cocoa powder
What is Cocoa Alkalization?
Cocoa alkalization is a value-added process in cocoa processing to produce alkalized cocoa mass or powder. It involves the treatment of cocoa nibs with a food-grade alkali solution to raise the pH, producing darker colors and stronger flavors.1
Alkalized cocoa mass or powder is used in chocolates, cakes, confectionery and beverages to generate desirable and unique characteristics. Reasons for alkalizing cocoa include:
- Reducing the acidity of natural cocoa (pH is raised from 5.2–5.6 to almost neutral values at 6.8–7.5)
- Reducing sourness
- Producing intense flavors and darker colors
- Increase solubility and dispersibility of cocoa in water (key in chocolate beverages)
Origin
The technique of alkalization was developed by Van Houten in the 19th century in Holland, thus the name ‘Dutching’ process. It involved treating cocoa nibs with alkali-potash to improve taste and color. Alkalized cocoa powders are still produced today and are preferred for many food and drink applications.2
How does it work?
The alkalization process can be applied to various cocoa products such as whole beans, nibs (before roasting and grinding), liquor (prior to pressing), or the powder, although it is most often the nibs that are alkalized.
The following block diagram shows various alkalization options in cocoa processing:
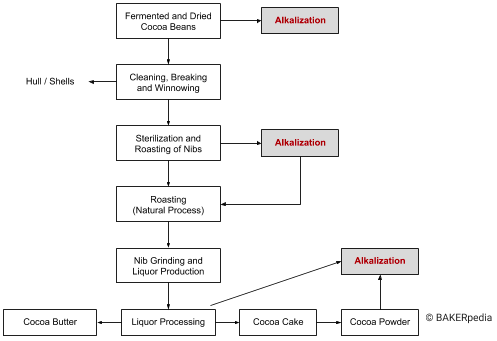
Alkalization of cocoa nibs (prior to grinding)
This is the traditional method and is considered to give a superior product (other alkalization possibilities require more complex conditions and may lead to undesirable lipid reactions). Nib alkalization can be carried out in a drum reaction vessel (pressurized) and/or in a screw conveyor once the unit is charged with the nibs.3
Processing steps:1,2
- Mixing/soaking. Mixing of nibs (6–7% moisture and at least 52% fat) and alkaline solution (sodium bicarbonate and water)
- Heating. Heating of mixture for sufficient time for color changes to take place. Depending on the cocoa beans used and processing conditions (e.g. concentration of alkaline solution, pressure and time), a specific color is produced.
- Drying. Once heating step is complete, the mixture is then dried at a temperature below 100°C (212°F) and roasted.
- It is also possible to alkalize and roast using the same piece of equipment (roasting drum).
Application of alkalized cocoa in cake systems
Dutched cocoa has been traditionally used in the production of premium Devil’s Food Cakes. Alkalized or Dutched cocoa has different baking characteristics and functionality. The following table summarizes and compares the acidity and color characteristics of some cake ingredients and the different alkalized cocoa that can be used:
| Cake ingredients | pH | Color |
| Chlorinated cake flour | 4.5–5.2 | Cream-white |
| Baking soda | 8.9–9.0 | White |
| Baking powder | 7.2–8.0 | White |
| Natural cocoa | 5.2–5.6 | Light brown |
| Cocoa, light Dutched or alkalized | 6.0–6.5 | Brown |
| Cocoa, medium Dutched | 6.5–7.0 | Reddish to dark brown |
| Cocoa, heavily Dutched | 7.0–8.0 | Red-brown to black (“ebony”) |
As a general rule in baking applications the higher the degree of cocoa alkalization:
| Dutched cocoa | Cake formulated with Dutched cocoa |
|
|
FDA regulation
According to 21 CFR Part 163, permitted alkaline agents (added as such or in aqueous solution) include:
- Ammonium bicarbonate
- Sodium bicarbonate
- Potassium bicarbonate
- Potassium and sodium carbonate
- Sodium and potassium hydroxide
- Magnesium carbonate
- Magnesium oxide
The regulation states that for each 100 pounds of cacao nibs, the total quantity of alkali ingredients used cannot be greater in neutralizing value (calculated from the respective combined weights of the alkali ingredients used) than the neutralizing value of 3 pounds of anhydrous potassium carbonate.
References
- Kamphuis, H.J. “Production of Cocoa Mass, Cocoa Butter and Cocoa Powder.” Beckett’s Industrial Chocolate Manufacture and Use, 5th edition, John Wiley & Sons Ltd, 2017, pp. 50–62.
- Afoakwa, E.O. “Cocoa Processing Technology.” Cocoa Production and Processing Technology, CRC Press, Taylor & Francis Group, LLC, 2014, pp. 167–175.
- Stauffer, C.E. “Chocolate and Confectionery Coatings.” Fats and Oils, Eagan Press Handbook Series, AACC International, Inc., 1996, pp. 93–94.

