
How do reducing agents work?
During mixing, the gluten in the flour is stretched and pulled apart so that it can be reformed during proofing and baking to provide the needed strength and structure. Reducing agents act like mixing to reversibly break down gluten so that once they have been used up the gluten reforms. This mechanism is the opposite of oxidizing agents, which build up gluten. Reducing and oxidizing agents can be used separately, or a reducing agent can be used with a slow oxidizing agent to increase gluten breakdown early in the process and gluten reformation later in the process.
When reducing agents are used with fast oxidizing agents (like iodate or azodicarbonamide), they counteract each other. Bread dough requires a combination of:
- Strength
- Extensibility
- Tolerance
These depend mostly on:
- Flour quality
- Water absorption
- Mixing conditions.
Reducing agents are used especially with high strength flour and high-speed processes to reduce mix time, lower energy input, improve machinability, and improve loaf volume.
Frozen bread dough is a special case where short mix time is especially important because it helps improve yeast stability. Extensibility is important in other yeast and chemically leavened applications including pizza, tortillas, cookies, saltines, and other crackers. Reducing agents decrease the elasticity that can cause shrinkage or curling after these products are formed.
What are the characteristics?
The characteristics of reducing agents depends on the source
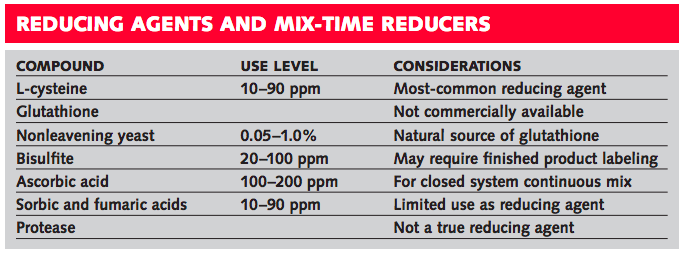
Protein-based reducing agents include cysteine, glutathione, and yeast. Cysteine is the most commonly used reducing agent in bread. It is an amino acid that is usually produced synthetically as L-cysteine hydrochloride, is usually added at the mixer, and acts quickly. Glutathione is a peptide that contains cysteine but is not generally available in its pure form. It functions similarly to L-cysteine but is potentially more effective because it can react more times. Yeast is a natural source of glutathione. Special non-leavening yeasts are used as reducing agents in the same applications as L-cysteine.
Sulfites are commonly used reducing agents in cookies and crackers. Their active ingredient is the bisulfite ion that is obtained from sulfur dioxide or from one of its salts, such as sodium bisulfite. Sulfites destroy the vitamin thiamine, are inhibitory to yeast, cause sensitivity reactions in some people, and require special label declarations if used in the U.S. at levels in the finished product above 10 parts per million.
Ascorbic acid (vitamin C) is used as a reducing agent only in certain closed continuous mix applications. In the presence of oxygen it functions as an oxidizing agent, but in the absence of oxygen, as a reducing agent. It can be used in coated form for increased stability as a component of bread improvers and dough conditioners.
Other acids that have been suggested as reducing agents, but are not commonly used, include sorbic acid and fumaric acid. They are part of a group of “activated double-bond compounds” and are more commonly used as preservatives. These acids are inhibitory to yeast and less economical than other synthetic reducing agents.
Proteases are used to decrease mix time and increase elasticity but are not reducing agents. They are natural enzymes that break down gluten irreversibly and must be used with careful attention to dose, time, and temperature to avoid over conversion.
Dough Reduction Chemistry
Now that we’ve covered what dough reducers do, let’s look at how they do it.
Case study: Cysteine as a dough reducer
Cysteine is unique among the protein amino acids because it has a sulfhydryl group at the end of the molecule. Cysteine is important to dough reduction chemistry because it occurs in the gluten protein from flour, in the tripeptide glutathione from yeast, and in free amino acid form as a synthetic reducing agent.
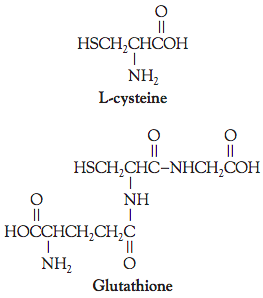
The significance of cysteine’s sulfhydryl group is that two of them from different proteins can be oxidized to one molecule of cystine, with the creation of a disulfide bond between them. When gluten molecules become linked (oxidized) during breadmaking, the dough strength increases but its extensibility decreases. During mixing, these linkages are broken mechanically to provide the extensibility needed for moulding. The process is reversible, and the gluten matrix reforms during the later stages of proofing and baking.
![]()
The disulfide bonds in gluten that are broken mechanically during mixing can also be broken chemically by a series of reactions with cysteine or glutathione known as disulfide interchange. The reactions are shown here, with R and R’ representing the two gluten molecules and with cysteine as the reducing agent:
R–S–S–R’ + cys–SH R–S–S–cys + R’–SH
R–S–S–cys + cys–SH cys–S–S–cys + R–SH
These reactions reduce the number of cross-links between the gluten subunits proportional to the number of cysteine or glutathione molecules added and are reversible so that the degree of relaxation can be controlled. The reaction is similar with either cysteine or glutathione, except that with glutathione less is required. This is because an enzyme present in flour converts glutathione disulfide into two glutathiones with free SH groups that can participate in further disulfide reactions. Because no analogous enzyme exists for cysteine sulfide, each cysteine SH can be used only once.

