
Lecithin
What is Lecithin?
Lecithin is a natural emulsifier and stabilizer in foods. It’s widely used in cakes and yeast-leavened bakery products. This ingredient is found in raw materials such as eggs or soybeans, and can be used as a clean label ingredient.
It is used as a:
- Wetting agent
- Pan release agent
- Cake batter stabilizer
- Fat replacer
Origin
Lecithin is a key component of cell membranes, and found frequently in nature. For example, it’s in plant sources such as soybeans, corn and rapeseed. Also, it’s found in animal products such as egg yolks.
It was discovered in 1846 by the French chemist Maurice Gobley. He isolated an orange-colored substance from egg yolk and called lecithin after the Greek name for egg yolk, “lekithos.”3 Egg yolk contains 10–20% lecithin, while most vegetable oils contain 0.1–3.0%.2
Learn more about types of lecithin and application tips in our free technical paper! Download here.
Function
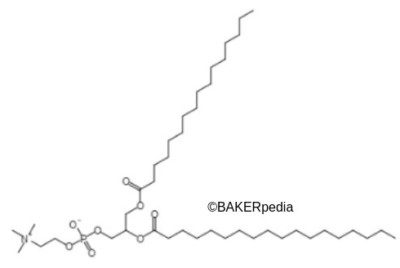
Chemically, lecithin is a glycerol with two fatty acid chains at carbons 1 and 2. Also, there is a phosphate ester group at the 3 position, which is bound to a molecule of choline. The phosphate and choline groups form phosphatidylcholine. This means the polar headgroup is the water loving part of lecithin.6 Meanwhile, fatty acid chains form the lipophilic region. These moieties make it an emulsifier.1,2
Lecithin’s hydrophilic-lipophilic balance (HLB) is intermediate and ranges from:7
- 3-4 for native fluid lecithin
- 7-8 for de-oiled lecithin
- 8- >10 for modified lecithin
This balance allows it to stabilize oil and water or water and oil emulsions and air bubbles in batters and creams. It also allows it to act as an effective wetting agent.2
Commercial production
Lecithin is produced commercially as a by-product of crude oilseed refining. It is a part of the “gum” that is removed during the degumming step of sunflower and rapeseed oil refining. To create different commercial products, bleaching with hydrogen peroxide, hot drying and chemical modifications are used.2
It is available as a powder or in liquid forms.
Application
Lecithin is used in baked goods, confections, chocolate, cocoa powder, dairy products, icings, frostings, margarine and other spreads. It is usually added to bread formulations at 0.2% and to layer cakes at 0.5–1.5%, based on flour weight. It is also used in cake donut dry mixes at 0.25–0.5% of total mix weight. As a result, its easier for wetting the dry blend when the batter is mixed. Lecithin provides:
- Finer crumb grain
- Greater loaf volume
- Better gluten stability
- Better emulsification of fats
- Longer shelf-life
- Increased water absorption
Lecithin has limited anti-staling ability, due to the molecule’s bulky structure. However, cleaving one fatty acid or the phosphatidylcholine moiety using lipases can improve its functionality as crumb softener.4
Handling considerations
Native lecithin is a sticky, viscous paste that can be difficult to weigh, handle and disperse in water. So, chemical modification such as hydroxylation or enzymatic treatment can improve its dispersibility in cold water, batters and doughs.1,2
FDA regulation
Lecithin is GRAS and can be used in food with no limitation, other than current GMP.5
Because of GMO concerns, some bakers choose to use lecithin from non-soy sources such as rapeseed oil. Or, more commonly, sunflower oil.
References
- De Leyn, I. “Other Functional Additives.” Bakery Products Science and Technology, 2nd edition, John Wiley & Sons, Ltd., 2014, pp. 295–306.
- Stauffer, C.E. Emulsifiers, Eagan Press Handbook Series, AACC International, Inc., 1999, pp. 25–66.
- Bueschelberger, H-G.. “Lecithins.” Emulsifiers in Food Technology, 2nd edition, John Wiley & Sons, Ltd., 2015, pp. 21–60.
- Msagati, T.A.M. “Emulsifiers.” Chemistry of Food Additives and Preservatives, John Wiley & Sons, Ltd., 2013, pp. 33–62.
- U.S. Food and Drug Administration, CFR – Code of Federal Regulations Title 21, Part 184: Direct Food Substances Affirmed As Generally Recognized As Safe, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=184. Accessed 25 May 2019.
- Pardun, Hermann, Die Pflanzenlecithine, Verlag für Chem. Industrie, 1988, p81ff.
- Ed. Szuhaj, Bernard F., Lecithins sources, Manufacture&Uses, AOCS, 1988, p 239.

