
Olestra
Also known as Olean
What is Olestra?
Olestra is a non-absorbable fat substitute that provides no calories to food products. It is made from a sucrose polyester and has a similar structure to fats. Olestra is commonly used in low-fat snacks, ready-to-heat popcorn, potato chips, and baked goods.1
Benefits of Olestra in food products include:
- High temperature stability
- Rich taste and creamy texture
Chemical Structure
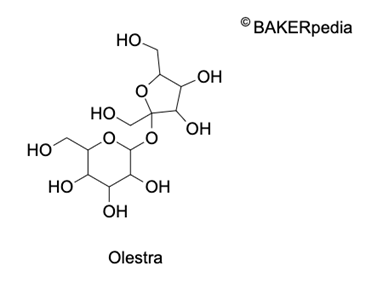
Origin
Olestra was originally discovered in 1968 by chemists Mattson and Volpenhein with Procter and Gamble and was patented in 1971. With increased consumer interest in low fat diets, and gaining FDA approval, several new Olestra-based low calorie products were launched in the US.1
Despite its usage in the US, Olestra is still banned in Canada and Europe due to some undesirable health concerns.1
Function
As a fat substitute, olestra provides similar functions to fat in baked goods, such as:2
- Tenderizer: by coating structure building components such as gluten, and starch, thus preventing their excessive hydration
- Moistness: by providing the sensation of moistness
- Prevents staling : via retarding starch gelatinization
- Smoothness: by interfering with sugar crystallization and enhancing smooth sensation
- Release agent: aids in the un-molding of baked goods
- Flavor: provides a similar flavor profile to traditional fats
Nutrition
Olestra is a no-calorie food additive and may help reduce cholesterol. Adverse effects associated with the consumption of this ingredient are decreased absorption of fat soluble vitamins and carotenoids. So, food products made with Olestra should include higher doses of these vitamins. It may also have a laxative effect in some individuals.1
Commercial production
Olestra is commercially produced through the following process:
- Transesterification: fatty acid methyl esters (FAMEs) are reacted with sucrose in the presence of potassium soaps in a reactor to produce a homogenous melt. Excess FAME is added with NaH followed by heating up to 130 – 150 oC (266 – 302oF) .
- Distillation: methanol is distilled from the reaction solution.
- Neutralization: the crude product is neutralized with acid addition.
- Washing: product is washed with warm water and alcohol.
- Bleaching: removal of pigments and other contaminants with bleaching clay.
- Distillation: for solvent removal.
- Deodorization: removal of undesirable odours.
- Addition of antioxidants for storage.
Common sources of triglycerides used in the manufacture of Olestra are cottonseed oil and soybean oil.
Application
Olestra can be used in a variety of applications and food formulations. Following is a list of some of these applications:1
- Cooking oil replacement up to 75%, due to its high heat stability.
- Fat substitute in baked goods such as: cookies, cakes, bread, among others. Providing similar textural and flavor properties.
- Snacks and potato chips
- Dressings and toppings
Textural and flavor profiles of Olestra’ are dependent on the original fatty acid used for sucrose esterification.
Regulations
The FDA considers Olestra a safe food additive for direct addition to food products, when following the condition established by the regulation.4
Olestra is banned in Canada and the European Union.5
References
- Roller, S and Jones, S.A. Handbook of fat replacers. CRC Press, 1996.
- Figoni, P. How Baking Works: Exploring The Fundamentals Of Baking Science. 2nd ed., John Wiley & Sons, Inc., 2008.
- Akoh, C.C. Food lipids: chemistry, nutrition, and biotechnology. CRC press, 2017.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 172 Food Additives Permitted For Direct Addition To Food For Human Consumption, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.867 , Accessed 08 August 2021.
- European Commission (EC). Commission Regulation (EU) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives . Official Journal Of European Communities, 16 December 2008.

