
Glycerin
Also known as glycerol, 1,2,3-Propanetriol
What is Glycerin?
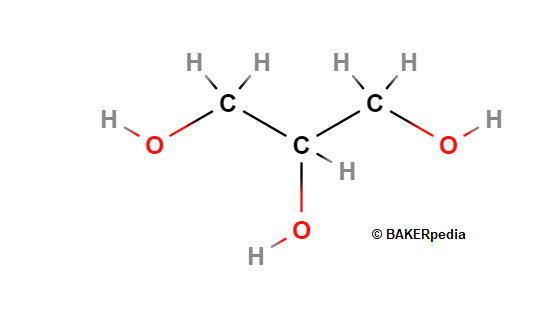
Glycerin is a three-carbon chain molecule which has an alcohol group bound to each carbon. It is a sweet and viscous liquid used in bakery foods as a humectant, water activity reducer and crystallization modifier. In food chemistry, glycerin is the backbone of fats and oils i.e mono-, di- and triglycerides.
Glycerin can form links with acid moieties (i.e. fatty acid chains) at the hydroxyl groups, losing a hydrogen and esterifying with these fatty acids to form fats.
- If each of the three arms of glycerin is attached to a fatty acid, the resultant product is a triglyceride.
- If only two are linked, it is a diglyceride.
- If only one is so linked, it is a monoglyceride.
Origin
Glycerin, or C3H8O3 is a naturally occurring substance which makes up the building blocks of fats, mono- and diglycerides, polyglycerol esters and many other food molecules. It can be obtained from microbial fermentation or via chemical synthesis.
Function
Glycerin is a multifaceted ingredient. The main functions of this molecule include:
- Carrier/solvent for flavor extracts and color mixes
- Sweetener
- Modifier of water activity (aw) in foods
- Shelf-life extender
- Humectant (used to maintain a certain moisture content to prevent the drying-out of foods)
- Crystallization modifier
- Plasticizer
Glycerin is used in confections to maintain the initial level of crystallization of the soft sugar. It also helps prevent ice crystal formation in frozen batter and dough.
Commercial production
Glycerin can be obtained from either natural sources (plants and animals) as well as industrial sources such as the by-products of biodiesel and soap. Alternatively, it can be obtained through chemical synthesis.
For glycerin to be suitable for human consumption, it must be ultra-pure (99.5%). The high purity standard for glycerin as a humectant food additive requires that the contents of ethylene glycol and diethylene glycol not exceed the limits of 0.1% in the finished product, set by the FDA.1
Once produced, glycerin is a clear, or yellowish hygroscopic viscous liquid with slight odor and slightly sweet taste.
Application
High-speed cakemaking has historically relied on the addition of glycerin and other polyols (e.g. propylene glycol) to obtain aw values as low as 0,85 to 0.80. In such applications, glycerin helps extend the microbial stability and keep a tender and moist mouthfeel in muffins, batter cakes, cake doughnuts, and many other sweet baked goods. Glycerin is also used in non-aerated icings.
Glycerin is usually added to batter cake formulations at levels ranging from 2.0 up to 15.0 % (based on flour weight). The higher the glycerin dose, the more tender the product and more prone to post-baking collapse due to insufficient starch gelatinization and crumb setting. Alternatives to glycerin include sorbitol, propylene glycol and other polyols.
Relevant properties of glycerin2
- Solubility: miscible in water and alcohol at 20°C (68°F)
- Molecular weight: 92,1 Da
- Boiling point at standard pressure (101,3 KPa / 0 feet above sea level): 290°C (554°F)
- Melting point: 18°C (64°F)
- Density in aqueous solution at 20°C (68°F): 1230 Kg/m3 (max. water content 5% w/w)
- Relative sweetness: approximately 65% (compared to sucrose)
Regulation
In the US, glycerin has a GRAS status when used in accordance with GMP. It can be used in alcoholic beverages, cured meats, and soft drinks. The FDA allows the addition of this ingredient without limit in all bakery goods.
Glycerin has the additives list number E-422. According to the European Food Safety Authority (EFSA) reports, glycerin is not an additive that has been subject to concerns regarding safety.
In the UK glycerin is allowed without limit in flour and baked goods but is prohibited in breads. GMP in cured meats, ices, soft drinks.
References
- Msagati, T.A.M. “Humectants.”Chemistry of Food Additives and Preservatives, John Wiley & Sons, Ltd., 2013, pp. 162–166.
- Moriartey, S. “Solvents.” Food Additives Data Book, 2nd edition, Blackwell Publishing Ltd., 2011, pp. 938–940.

