
Calcium Propionate
What is Calcium Propionate?
Calcium propionate is the calcium salt of propionic acid. It is a preservative commonly used in baked goods around the world, where it extends their shelf life by inhibiting the growth of spoilage microorganisms, namely mold and ropy bacteria.
Characteristics of calcium propionate include:
- Chemical formula: C6H10O4 Ca
- Molecular Weight: 186.22
- Works best at pH below 5.5
- Recommended usage level in bakery: 0.1-0.3% flour weight, but higher levels not uncommon
- Nutrition: 21 grams of calcium are present in 100 grams of calcium propionate5
Chemical structure
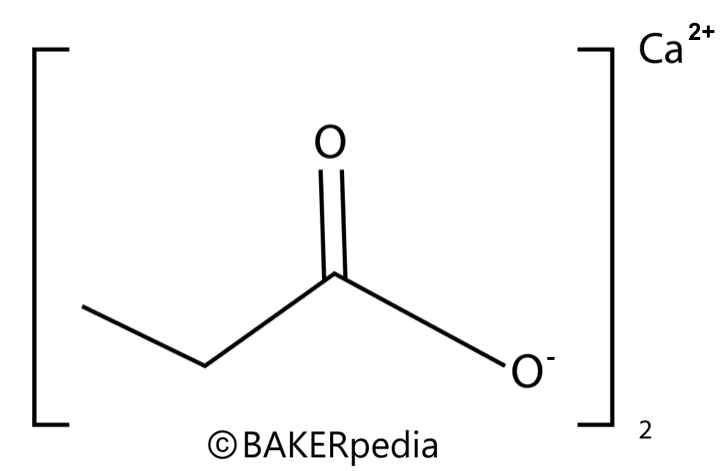
Chemical structure of calcium propionate.
Origin
Propionic acid occurs naturally in swiss cheese at 1%1. Together with its calcium salt derivative, it has been established as a bakery antimicrobial for a long time. As early as 1906, Watkins recognized that it was effective against ropy bacteria in bread.2 Since the 1930s, propionates have been widely used to preserve bread in the United States.
Is calcium propionate safe?
Function
Calcium propionate is commonly used as a preservative in yeast-raised baked products such as pre-packed and sliced bread, and in some chemically-leavened goods like tortillas. It is added during the dough phase and its optimal use level is mainly dependent on the formula and the desired shelf life of the finished product.
Commercial production
Calcium propionate is formed by neutralizing chemically-synthesized propionic acid with calcium hydroxide. Although it has been a preservative of choice in bakery for decades, in recent years it has been increasingly challenged as non-clean label by many consumers and leading retailers,6,7 resulting in a push to reduce or altogether eliminate it from formulations.
Application
Here are some factors that bakers and formulators must consider when using this ingredient:
Calcium propionate is most active in the pH range below 5.5. Therefore, it is common to use acids to adjust the pH to optimize the activity. Moreover, salts of benzoic or sorbic acid are recommended for use in products with higher pH levels, such as in many chemically leavened sweet baked goods. In tortillas, calcium propionate and potassium sorbate are commonly used together, to achieve a broad spectrum of mold inhibition while maintaining product quality.
Calcium propionate is the ideal preservative for bread and rolls because it has little effect on yeast and does not interfere with its fermentation. In some applications such as cakes, however, it may not be a good preservative option, as the high use level and its available calcium interfere with the chemical leavening.4 In contrast, sodium propionate will delay fermentation of yeast and is not recommended for use in breads or rolls, but it is preferred for the preservation of cakes.4
Calcium propionate is effective at inhibiting growth of mold and ropy bacteria when its dose relative to the number of microbial cells present is adequate to block cell metabolism. If the baked good is produced in an environment without effective current good manufacturing practices (cGMP), the dose may not be effective in inhibiting microbial growth.
Regulations
In the United States, calcium propionate is affirmed as a Generally Recognized as Safe (GRAS) food substance under the following conditions:
- It is used as an antimicrobial agent
- It is used in accordance with current good manufacturing practices and use does not exceed what is needed for desired effect
- It is used in baked goods, cheeses, confections, frostings, gelatins, puddings, fillings, jams or jellies.
- Meets the specifications of the Food Chemical Codex (FCC) 3rd edition. (21 C.F.R. § 184.1221 2018)
The European Food Safety Authority re-evaluated calcium propionate (E-282) as a food additive in 2014 and found no safety concerns for this additive.1 Maximum permitted level (MPL) is specified in Annex II of Regulation (EC) No 13333/2008 on food additives, and the panel does not have any concerns at this level or in these applications:
| Category | Foods | Restrictions | MPL or mg/Kg |
| 07.1 | Bread and rolls | Only pre-packaged sliced bread and rye bread | 3000 |
| 07.1 | Bread and rolls | Only energy reduced bread, partially baked pre-packed bread, and pre-packaged roll and pita, prepackaged polsebrod, bolle and dansk flutes | 2000 |
| 07.1 | Bread and rolls | Only pre-packaged bread | 1000 |
References
- “Food Additives”, Food Chemistry 2nd Edition, ed. Owen Fennema. Marcel Dekker, New York. 1985.
- Luck, E, Jager, M. “Propionic Acid”, Antimicrobial Food Additives. Springer, London. 1997.
- EFSA. “Scientific Opinion on the re-evaluation of propionic acid (E 280), sodium propionate (E 281), calcium propionate (E 282) and potassium propionate (E 283) as food additives. EFSA Journal.12:7:3779.. 2014.
- Chemical Book, Calcium propionate. 2017. www.chemicalbook.com/ChemicalProductProperty_EN_CB6194031.htm. Accessed 1 February 2019.
- Macco. Nutritional Data Calcium Propionate. 2012. www.macco.cz/ca/resources/pdf-data/37E_CalPro-Nutridata.pdf. Accessed 1 February 2019.
- Whole Foods Market. “Unacceptable Ingredients for Food (as of March 15, 2019).” https://assets.wholefoodsmarket.com/www/products/quality-standards/Unacceptable_Ingredients_for_Food_031519.pdf Accessed 15 Sept 2020.
- Panera Bread. “The No No List, April 16, 2018” https://www.panerabread.com/panerabread/documents/panera-no-no-list-05-2015.pdf Accessed 15 Sept 2020.

