[fusion_builder_container hundred_percent=”yes” overflow=”visible” type=”legacy”][fusion_builder_row][fusion_builder_column type=”1_2″ layout=”1_2″ last=”false” spacing=”yes” center_content=”no” hide_on_mobile=”no” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_color=”” border_style=”solid” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”0px” animation_type=”0″ animation_direction=”down” animation_speed=”0.1″ animation_offset=”” class=”” id=”” border_sizes_top=”0px” border_sizes_bottom=”0px” border_sizes_left=”0px” border_sizes_right=”0px” first=”true” spacing_right=”2%” min_height=””][fusion_text]

Baking powder is a leavening ingredient used in a variety of recipes for baked goods.
[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_2″ layout=”1_2″ last=”true” spacing=”yes” center_content=”no” hide_on_mobile=”no” background_color=”” background_image=”” background_repeat=”no-repeat” background_position=”left top” hover_type=”none” link=”” border_position=”all” border_color=”” border_style=”” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”” margin_bottom=”” animation_type=”” animation_direction=”” animation_speed=”0.1″ animation_offset=”” class=”” id=”” border_sizes_top=”0px” border_sizes_bottom=”0px” border_sizes_left=”0px” border_sizes_right=”0px” first=”false” spacing_left=”2%” min_height=””][fusion_text]
Baking Powder
Also known as Chemical Leavening, Double-Acting Baking Powder
What is Baking Powder?
Baking powder is a chemical leavening agent. Chemical leavening involves the action of an acid on bicarbonate to release carbon dioxide gas for aeration of a dough or batter during mixing and baking.1
Baking powder contains sodium bicarbonate, a leavening acid, and cornstarch. It becomes active when water is added to a formula.[/fusion_text][/fusion_builder_column][fusion_builder_column type=”1_1″ layout=”1_1″ background_position=”left top” background_color=”” border_color=”” border_style=”solid” spacing=”yes” background_image=”” background_repeat=”no-repeat” padding_top=”” padding_right=”” padding_bottom=”” padding_left=”” margin_top=”0px” margin_bottom=”0px” class=”” id=”” animation_type=”” animation_speed=”0.3″ animation_direction=”left” hide_on_mobile=”no” center_content=”no” min_height=”none” align_self=”flex-start” border_sizes_undefined=”” first=”true” last=”true” hover_type=”none” link=”” border_position=”all”][fusion_text]
Origin
In 1856, chemist Eben Norton Horsford patented the first modern baking powder.2 Horsford originally extracted monocalcium phosphate by boiling animal bones. The monocalcium phosphate acted as an acid that combined with baking soda to create a reaction producing C02.
By the 1880s, Horsford’s company switched to mining the monocalcium phosphate to lower costs.2 He placed the two ingredients together in one container, adding cornstarch to soak up moisture, which prevented the ingredients from reacting prematurely.2 The company he created became known as Rumford.
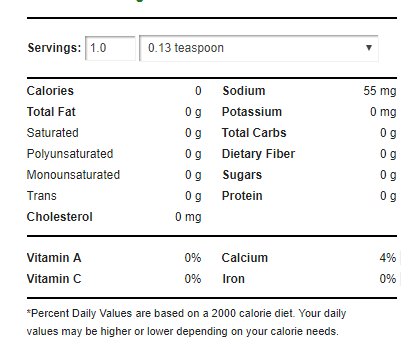
Nutrition
Baking powder has the following nutritional panel:3
Function
Baking powder, when hydrated, will aerate the dough or batter. The aeration provides a light, porous cell structure, fine grain, and a texture with desirable appearance, along with palatability, to baked goods.1
Commonly used leavening acids in baking powder are MCP, SAPP, SALP, SAS, and tartaric acid. These may be used individually or in combination. MCP is fast-reacting so it is often combined with a slower-acting leavening acid, like SAPP, SALP, or SAS. An example reaction is shown below:
NaHCO3 + H+ = Na+ + CO2 + H2O
Sodium bicarbonate Acid Sodium Carbon Dioxide Water
Baking powder is classified according to its reaction rate:4
- Fast-acting types release most of their potential gas volume during the first few minutes of contact with liquid, thus imposing the need for fairly rapid processing of the dough or batter to avoid excessive volume loss.
- Slow-acting types release almost none of their gas volume at low temperatures and require oven heat to achieve complete reaction.
- Double-acting types react partially at low temperatures to form smooth-flowing batters, but typically require high temperatures for complete reaction.
Fillers are used in addition to the active components to make baking powder shelf-stable. The most common filler is corn starch. Corn starch may be augmented with calcium carbonate, calcium sulfate, calcium lactate, or calcium silicate.4 Fillers perform a two-fold function:
- Stabilize the product by keeping the active ingredients apart, thereby preventing premature reaction, in case moisture should find access to the powder4
- Standardize the strength of the powder4
Commercial Production
Baking powder production begins with the raw ingredients.
The process is as follows:5
- Sodium carbonate is produced using the Solvay ammonia process. Ammonia and carbon dioxide are passed through a saltwater solution in an absorption tower. This results in a compound called ammonium bicarbonate, which reacts with the salt to produce sodium bicarbonate crystals and ammonium chloride.
- Bicarbonate crystals are filtered out by centrifuges and washed to remove residual chloride. The material is heated and reacted with carbon dioxide to produce sodium carbonate.
- Sodium carbonate is then dissolved, carbonated, and cooled, which results in crystallized sodium bicarbonate.
- Tartaric acid is made using potassium hydrogen tartrate, which is a waste product from wine-making.
- Tartaric acid and sodium bicarbonate are blended and cornstarch is added. Industrial mixers combine the powders into a single, homogeneous blend.
Application
Baking powder may be single- or double-acting. Double-acting is more common and means that the baking powder contains a combination of leavening acids that will release gas during the mixing and again during baking.
There are a variety of baking powders on the market, allowing for use in a wide range of applications. Things to consider when choosing a baking powder are reaction time, single- vs. double-acting in relation to the processing time, and baking temperature/time for the product. Baking powder will also impact the finished product’s pH.
When creating a recipe, the formula ratio is 1 to 1 ¼ teaspoon of baking powder per 1 cup flour.6 Using too much baking powder will result in a bitter-tasting product, large bubbles, and surface blistering. Using too little will result in tough dense crumb with little volume.
Although baking powder has a shelf life of 24 months from date of manufacture it may lose efficacy within 6 months of opening.6 A quick test can determine the potency. Stir 1 teaspoon baking powder into 1/3 cup of hot water.6 If bubbles appear, it is fine to use.
You can make your own baking powder by combining 15ml/1 tbsp bicarbonate of soda with 30ml/2 tbsp cream of tartar.6
FDA regulations
CFR Title 21 Sec. 182.1 lists baking powder as generally recognized as safe (GRAS) when used in accordance with good manufacturing practices.7
References
- Chung, F.H.Y. “Bakery Processes, Chemical Leavening Agents.” Wiley Online Library. John Wiley & Sons, Inc., 4 December 2000. http://onlinelibrary.wiley.com/doi/10.1002/0471238961.0308051303082114.a01/abstract. Accessed 22 August 2017.
- Panko, B. “The Great Uprising: How a Powder Revolutionized Baking.” Smithsonian.com. Smithsonian Institution, 20 June 2017. www.smithsonianmag.com/science-nature/great-uprising-how-powder-revolutionized-baking-180963772/. Accessed 22 August 2017.
- “Calories in baking powder.” Baking Powder Nutrition Facts, Baking Powder Calories, Nutritional Information. Under Armour. www.myfitnesspal.com/nutrition-facts-calories/baking-powder. Accessed 22 August 2017.
- Pyler, E.J. Baking Science & Technology. 3rd ed., vol. 2, Sosland, 1988. 931-933.
- “Baking Powder.” How Products Are Made. www.madehow.com/Volume-6/Baking-Powder.html. Accessed 22 August 2017
- “Baking Powder.” What’s Cooking America. 15 December 2016. https://whatscookingamerica.net/Q-A/BakingPowder.htm Accessed 22 August 2017.
- US Food and Drug Administration. “CFR – Code of Federal Regulations 21CFR182.1.” 1 April 2016. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.1. Accessed 18 August 2017.
[/fusion_text][fusion_text]
 [/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]
[/fusion_text][/fusion_builder_column][/fusion_builder_row][/fusion_builder_container]