
It is no secret that, if used properly, the application of the transglutaminase enzyme (TG) in bakery products can promote great improvement in dough texture, volume, and gas retention, even when combined with other enzymes.
Transglutaminases (EC 2.3.2.13) γ-glutamyl-peptide, amine-γ-glutamyl-transferase) belong to the group of acyltransferases, which catalyze acyl-transfer reactions between a γ-carboxyamine, group of a peptide – or protein-bond glutamyl residue and a primary amino group of various substrates including the ε-amino group of lysine or lysyl residues in proteins, resulting in polymerization or amine incorporation.
The result cross-link is called a ε-(γ-glutamyl)lysine isopeptide bond). During the reaction a single molecule of ammonia is generated per cross-link. In case the amine substrates are not available as acyl acceptors, it is possible for TG to catalyse deamination of glutamyl residues using water as an acyl acceptor. In contrast to their limited glutamine (an acyl donor) substrate specificity, TG possesses a wide specificity for the acyl acceptor substrates (Fig. 1).
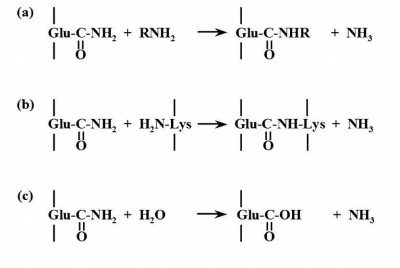
Figure 1 – Transglutaminase mode of action. a) Acyl transfer, b) Crosslinking of Gln and Lys residues in proteins or peptides, c) Deamidation.
Transglutaminase applications and effects:
| Application | Effect | Reference |
| Pastry product | Improved preservation and lift of puffy pastry | Gerrard et al, 2000 |
| Frozen, laminated doughs | Improved stability, texture and volume | Gerrard 2002, Pozza 2002 |
| Low quality flour baking | Rebuilding the structure of dough | Koksel et al 2001, Cabellero et al, 2005 |
| Noodles and pasta manufacturing | Improved texture | Wu and Cork, 2005 |
| Gluten-free baking | Formation of protein network, improved loaf volume, crumb | Moore et al, 2006 |
| Frozen dough | Stabilize the gluten structure embedded by starch granules | Huang et al, 2008 |
| Rice noodles | Cooking loss and water turbidity of rice noodle decreased | Kim et al, 2013 |
| Dough improvers | Improved volume, texture and shelf life of baked goods | Esteller, 2016 |
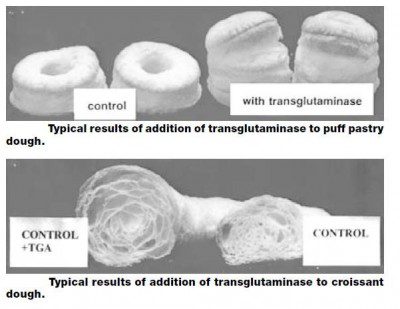
Fig. 2 – TG in croissant (from J.A. Gerrard et al., JFS, v. 65, n. 2, 2000).
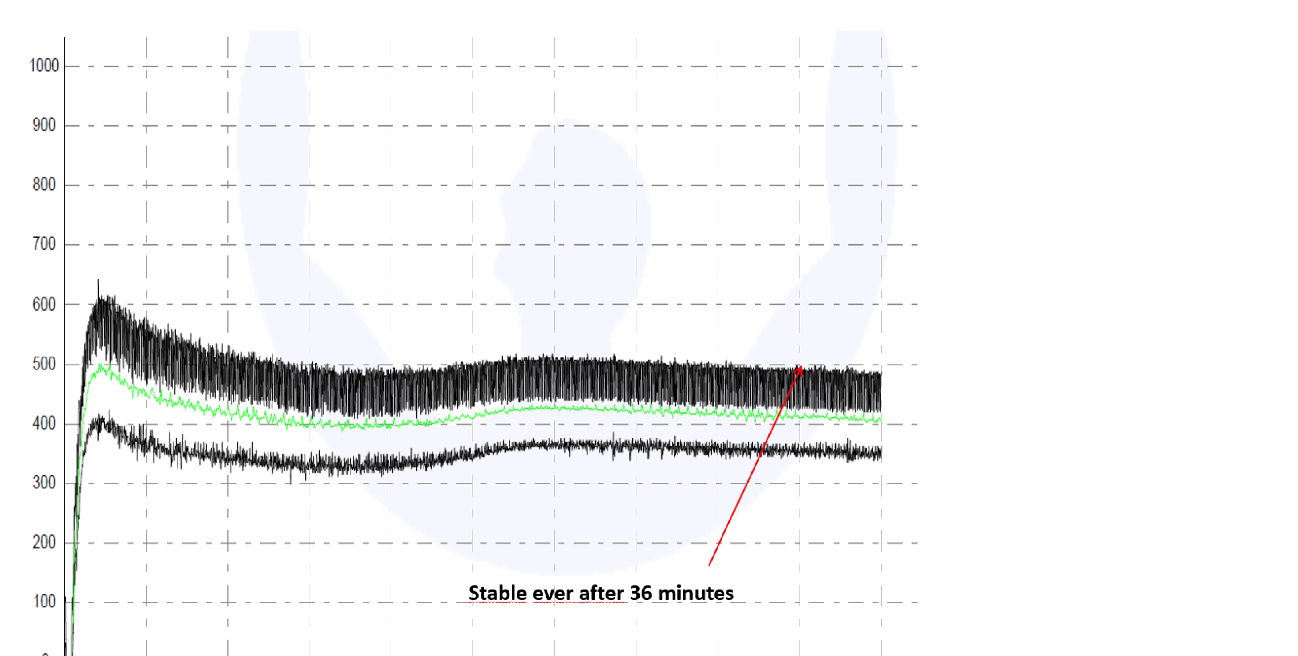
Fig. 3 – Addition of 0.03%/300g wheat flour with concentrated and encapsulated transglutaminase corresponding to 25-35 u/g (Mauricio Esteller, 2016).
So what’s new?
What few people know, however, is the deleterious effects of oxygen (it can oxidize sulfhydryl groups) on the stability of the transglutaminase enzyme and the consequent reduction or loss in activity during storage and manipulation.
Most well-known manufacturers tend to store TG in vacuum packages and refrigerated storage, which causes many setbacks after the package is opened. It needs to be fractionated over successive applications if vacuum pumps or barrier films are not available.
How to solve the problem?
By using an innovative technology which concentrates and encapsulates the protein this issue can be avoided without affecting performance of the transglutaminase enzyme in industrial processing or long storage of the dough improver. New trials with this new stable TG generation showed best improved effects.

