
Ascorbic Acid
Also known as vitamin C or dehydroascorbic acid (baking industry)
What is Ascorbic Acid?
Ascorbic acid, also known as vitamin C, is an essential nutrient found in citrus fruits and many vegetables. It is used as wheat flour improver in yeast-leavened baked goods to help increase the volume of bread and provide better tolerance to variable processing conditions, such as dough temperatures and proofing times.
Along with dough strengthening enzymes, such as lipase, amylase and xylanase, it is frequently used in clean label bread formulations.
Origin
Albert Von Szent Györgyi discovered ascorbic acid in the late 1920s and was awarded the Nobel Prize in 1937 for his discovery. At that time it was used as a cure for scurvy caused by a dietary deficiency in fresh fruits and vegetables.¹,²
In 1935 the use of ascorbic acid as a flour improver, or dough conditioner, was developed by Jorgensen. At doses as low as 20–30 mg per kilogram of flour bread volume increased by 20%.¹,²
Function
One prominent function of ascorbic acid in bread dough is to stabilize the gluten protein network. This is reflected in greater loaf volume and a finer, more uniform crumb structure.
Unlike traditional oxidizing agents, such as potassium bromate, azodicarbonamide (ADA) and calcium peroxide, ascorbic acid depends strongly on external factors. To function as an oxidizing agent, ascorbic acid requires both oxygen and the presence of an enzyme which occurs naturally in cereal grains.³
Chemical Structure
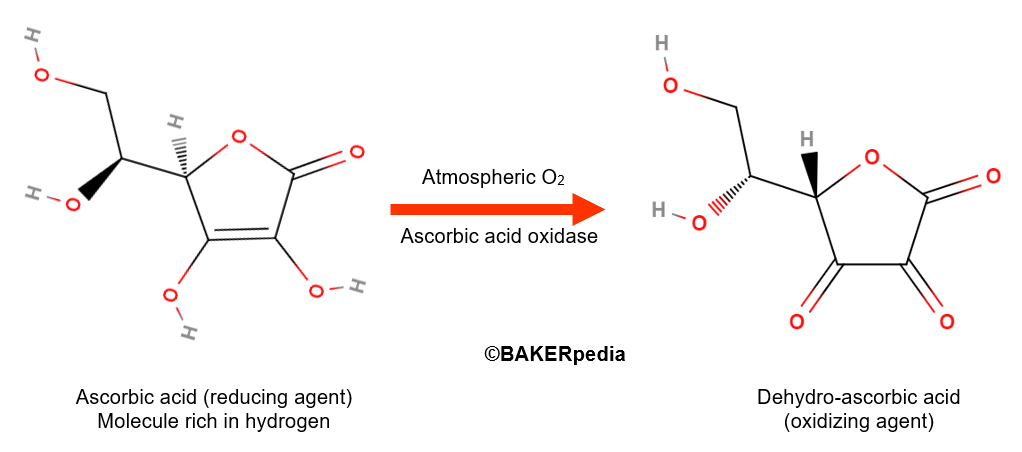 Ascorbic acid itself is a reducing agent with strong antioxidant properties in food systems. However, in the presence of oxygen gas and ascorbic acid oxidase, an enzyme naturally found in wheat flour, ascorbic acid is converted to its dehydro form as shown above.
Ascorbic acid itself is a reducing agent with strong antioxidant properties in food systems. However, in the presence of oxygen gas and ascorbic acid oxidase, an enzyme naturally found in wheat flour, ascorbic acid is converted to its dehydro form as shown above.
It is the oxidized form of AA that has the potential to take part in oxidation reactions during flour-water mixing, such as SH/SS interchange between cysteine residues of gluten-forming proteins. The oxidation of thiol groups promotes the formation of disulphide bonds between proteins, causing gluten cross-linking and polymerization (gluten strengthening effect).
Gluten strengthening can have various consequences in breadmaking:
- Higher gas retention capacity of dough
- Greater elasticity of dough
- Higher dough tolerance to over-proofing and over-mixing
- Higher water absorption of flour to obtain equivalent dough rheology after mixing
- Diminished dough viscous behaviour
- Higher dough resistance to deformation
- Enhanced oven spring during baking
Commercial production
Fruits and vegetables are considered natural sources of the micronutrient ascorbic acid, commonly known as vitamin C. Most of the ascorbic acid used in the food industry is synthesized from carbon sources (e.g. glucose or molasses) using a combination of microbial fermentations and chemical methods.
Application
Ascorbic acid is a white granular powder that is often used in bread and buns at levels up to 150 ppm (based on flour weight). The exact amount dosed of AA depends on the following factors:
- Protein level of flour
- Breadmaking system used (e.g. sponge and dough, no-time dough, Chorleywood bread process)
- Bake test results
- Scaling weight/pan volume ratio
- Amount of other oxidizing agents (e.g. ascorbic acid for ADA or bromate replacement)
- Amount of bran, whole grains and fruit inclusions in formulation (whole wheat and multigrain bread require a higher amounts of AA)
Factors that affect the oxidizing potential of ascorbic acid
- Amount and enzyme activity of ascorbate oxidase in flour
- Amount of atmospheric oxygen during mixing
- Competition with yeast and glucose oxidase for air (oxygen)
Regulation
According to the U.S. FDA, ascorbic acid is a dough conditioner and flour improver permitted to a maximum of 200 parts per million (based on flour weight).¹
In the UK and European Union, AA is permitted in all flour and breads except wholemeal to a maximum of 200 ppm.
References
- Grosch W., Wieser H. Redox reactions in wheat dough as affected by ascorbic acid. Journal of Cereal Science 29.1 (1999):1-16.
- Arrigoni O., De Tullio M.C. Ascorbic acid: much more than just an antioxidant. Biochimica et Biophysica Acta (BBA) – General Subjects 1569.1 (2002):1-9.
- Sahi, S.S. “Ascorbic acid and redox agents in Bakery systems.” Bakery Products Science and Technology, 2nd edition, John Wiley & Sons, Ltd, 2014, pp. 183–197.
- Federal Register: Electronic Code of Federal Regulations, Title 21, Chapter I, Subchapter B, Part 137—Cereal Flour and Related Products, https://ecfr.federalregister.gov/current/title-21/chapter-I/subchapter-B/part-137, Accessed 13 September 2020.

