
Ascorbyl Palmitate
What is Ascorbyl Palmitate?
Ascorbyl palmitate is a food additive used as an antioxidant. It is an ester of ascorbic acid (Vitamin C) and palmitic acid.¹,²
- In baking, ascorbyl palmitate is used as an antioxidant or preservant to improve product shelf life of fat-based systems, like frostings and fillings.
- It may also be used as a functional ingredient to enrich foods with vitamin C. ¹,²
Chemical Structure
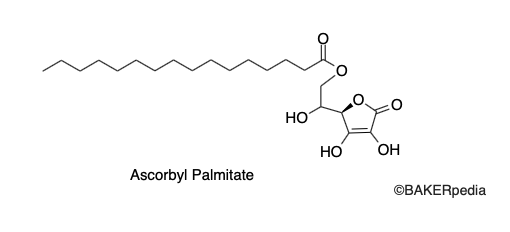
Origin
Ascorbyl palmitate (6-O-Palmitoyl- L -ascorbic acid) is obtained from the reaction of palmitic acid and L-ascorbic acid through chemical or enzymatic catalysis. It can also be obtained from the condensing reaction of palmitoyl chloride and ascorbic acid. ¹,²,³
Lloyd A Hall, an American chemist identified the antioxidant properties of ascorbyl palmitate and developed methods to use other antioxidants like citric acid, lecithin and propyl gallate to prevent food oxidation. ¹,²,³
Function
Ascorbyl palmitate has several functions in bakery products: ¹,²,³
- Oxygen scavenger or reducing agent: prevents oxidation by donating hydrogen
- Surfactant
- Shortening substitute
- Shelf life improver: retards autoxidation of lipids during storage
Benefits of ascorbyl palmitate in breadmaking include: ¹,²,³
- Increased water absorption (up to 4%)
- Increased dough strength and machinability
- Softening effect on bread crumb
- Increased bread volume
- Reversing detrimental effects of defatted soy flour proteins
Nutrition
Ascorbyl palmitate has similar health effects to ascorbic acid (Vitamin C). It is more bioavailable than ascorbic acid due to its liposoluble nature. Ascorbyl palmitate aids several physiological processes such as iron absorption and tissue repair. It can help decrease cancer and cardiovascular disease risk. ¹,²,³
Commercial production
Ascorbyl palmitate is commercially produced through the two following processes:
Chemical process: ²
- Synthesis: catalyzed esterification reaction of ascorbic acid with concentrated sulfuric acid in the presence of palmitic or stearic acid. The reaction occurs at high temperatures for around 10-24 h.
- Extraction: ascorbyl palmitate is extracted using solvents like ether, mesityl oxide or acetone.
- Purification and recrystallization to yield white to yellowish ascorbyl palmitate powder .
Enzymatic process:
- Synthesis: ascorbic acid and an organic acid enol ester react in the presence of an active lipase at 25 °C (77°F) and an organic solvent (pyridine, t-butanol, dioxane or tetrahydrofuran) with a solubility in ascorbic acid of more than 0.3% to form ascorbyl palmitate. ²
- Extraction: ascorbyl palmitate is extracted using suitable solvents.²
Application
Ascorbyl palmitate is commonly used in fat-based systems such as fillings and frostings. However, positive effects have been found in bread making, just like ascorbic acid.
Some considerations when working with ascorbyl palmitate: ¹,³
- Must be dispersed in oil or fat before being mixed with other ingredients.
- It can be added to bakery products in amounts ranging from 0.1 to 0.4% to retard staling for 2-4 days.
- The optimum absorption level is 68% when 0.38% ascorbyl palmitate is added to the dough.
- High levels above 0.4% may produce rigid dough that may not expand.
- It can be mixed with lecithin for a better effect
- It can be mixed with monoglycerides at a 9:1 ratio.
Levels of ascorbyl palmitate in some food products:¹
| Product | Usage level | Effect |
| Animal fat | 0.01-0.02% |
Combination with tocopherols and lecithin effectively retards oxidation in fats. A mix of 500 ppm ascorbyl palmitate and 100 ppm tocopherol decreases beef tallow oxidation. |
| Vegetable fat | 0.01-0.02% | Enhances shelf life at 0.01%. |
| Butter | 0.001-0.02% | antioxidant |
| Whole milk powder | 0.01-0.05% | Stabilizes up to 6 months at 0.5%. |
Ascorbyl palmitate is more effective than butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT) and propyl gallate (PG) in vegetable oils. ¹
Regulations
Ascorbyl Palmitate is considered GRAS by the FDA, when used under the appropriate good manufacturing practices.4
In the EU, ascorbyl palmitate (E 304) is regulated by EU Commission Regulation No 1925/2006.5
References
- Madhavi, Dl L., S. S. Deshpande, and Dattajirao K. Salunkhe. Food antioxidants: Technological: Toxicological and health perspectives. CRC Press, 1995.
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). “Scientific Opinion on the re‐evaluation of ascorbyl palmitate (E 304 (i)) and ascorbyl stearate (E 304 (ii)) as food additives.” EFSA Journal 13.11 (2015): 4289.
- Ensminger, Marion Eugene, and Audrey H. Ensminger. Foods & Nutrition Encyclopedia, Two Volume Set. CRC press, 1993.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 182 Substances Generally Recognized as Safe, https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.3149 . Accessed 14 September 2020.
- European Commission (EC). Commission Regulation NO1925/2006 of the European Parliament and of the Council of 20 December of 2006 on the addition of vitamins and minerals and of certain substances to foods . Official Journal of European Communities, 20 December 2006.

