Sodium Aluminium Phosphate (SALP)
Also known as Sodium Aluminium Phosphate Acid
What is Sodium Aluminium Phosphate?
Sodium Aluminium Phosphate (SALP) is a food additive and a leavening acid. It is a white to colorless powder and is considered a slow reacting leavening acid with a neutralizing value of 100.1,2 In baking, SALP is typically used in biscuits, muffins and sponge cakes.
Commercially available SALP can be found in two forms, both with similar neutralizing value:1,2
- Sodium Aluminium Phosphate hydrate
- Sodium Aluminium Phosphate anhydrous
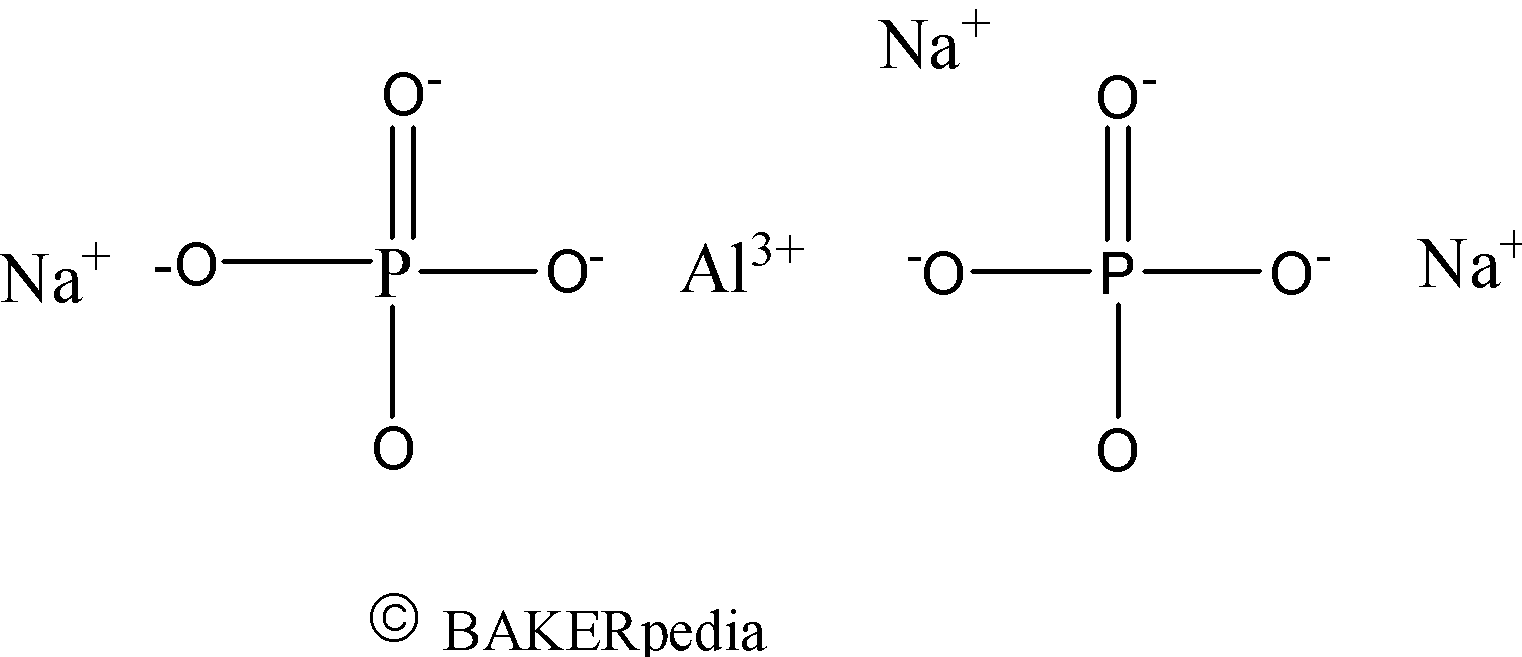
Chemical Structure
Origin
SALP is credited as the ingredient that made it possible to prepare quick cakes mixes which allow long standing periods of no reaction with baking soda. It is obtained from sodium hydroxide or sodium carbonate, a trivalent aluminum containing compound such as sodium aluminate or hydrated alumina, and a phosphorus compound like orthophosphoric acid or sodium orthophosphate.1,2
Function
SALP is used in baked products mainly as an acid leavening agent with the following functionality:1,2
- Reacts slowly even at high temperatures, thus its use when slow release of leavening gases is required.
- Has a bland taste.
- Increases dough tolerance to ingredients and flour variability.
- Improves tenderness and moistness of baked products.
- Improves cake volume.
Nutrition
SALP has an Acceptable Daily Intake (ADI) of 1 mg/kg body weight. Commonly used levels of SALP don’t exceed this value. High sodium content of SALP may impart potential health risks such as increased risk of heart disease.3
Commercial production
SALP is commercially produced by the following process:4
- Reaction mixture: sodium carbonate, aluminum hydroxide and phosphoric acid are mixed in a reaction vessel with a water content of around 30%. A metal sulfate is used to avoid early precipitate formation.
- Formation of sprayable solution by diluting the resulting mixture.
- Spray drying: using spray drying..
- Recovering the dried product
Application
As a leavening acid, SALP is commonly used to:1,2
- Flour mixes (cake, pancakes, biscuits and muffins)
- Frozen doughs
- Self-rising flours
- Sponge cakes
- Scones
- Double acting baking powders
- And generally when slow release or long bench times are required.
When working with SALP the following aspects should be taken into consideration:1,2
- It has a neutralizing value of 100.
- Requires high temperature to react and remains relatively stable during bench time.
- It is commonly used in combination with Monocalcium Phosphate (MCP) and Sodium Acid Pyrophosphate (SAPP).
- It releases around 20 – 30% of carbon dioxide (CO2) during resting periods, and the rest through baking.
- Gas release starts at around 40 – 43 oC (104 – 110 oF).
Typical usage levels of SALP can be seen in the following table:1,2
| Bakery Products | Usage level (flour basis) | Effect |
|---|---|---|
| Layer Cake | 1.7 -2.0% (as a mix of SALP, MCP and SAPP) |
|
| Corn bread and muffins | 1.5 – 2.0 % (as a mix of SALP, MCP and coated MCP) |
|
| Pancakes and waffles | 2.0 – 2.5% (as a mix of SALP, MCP and SAPP) |
|
| Refrigerated biscuits and doughs | 2.0 – 2.3% (as a mix of SALP, MCP and SAPP) |
|
| Biscuits | 1.4 – 2.0 % (as a mix of SALP, MCP and SAPP) |
|
Regulations
SALP is generally considered safe by the FDA when used for its intended use and following good manufacturing practices.5
In the EU, SALP (E 541) use is limited to scones, sponge cakes with jelly or jam filling. SALP is regulated by the EU Commission No 380/2012.6
References
- Ellinger, R. H. Phosphates as food ingredients. CRC Press, 2018.
- Smith, J, ed. Food additive user’s handbook. Glasgow: Blackie, 1991.
- Wood, Roger, et al. Analytical methods for food additives. Elsevier, 2004.
- Chiang, John SC. “Method of producing sodium aluminum phosphate.” U.S. Patent No. 4,375,456. 1 Mar. 1983.
- Food and Drug Administration (FDA). US Department of Health and Human Services. CFR Code of Federal Regulations Title 21, Part 182 Substances Generally Recognized as Safe ,https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=182.1781 , Accessed 19 December 2020.
- European Commission (Ec). Commission Regulation (Eu) No 380/2011 Cof 3 May 2012amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council as regards the conditions of use and the use levels for aluminium-containing food additives . Official Journal Of European Communities, 03 May 2012.