Baking Soda
Also known as sodium bicarbonate or bicarbonate of soda
What is Baking Soda?
Baking soda is one of the most commonly-used chemical leavening agents. It is an alkaline salt which requires heat and/or an acid to generate leavening gases.¹ Similar to other chemical leaveners, it is typically used in baked products that do not require yeasts such as cakes, cookies, muffins and cupcakes.
- Commercially, baking soda can be found in different particle sizes
- It ranges from 70 to 160 microns i.e. from fine powder to granular
- This affects its reactivity. ¹
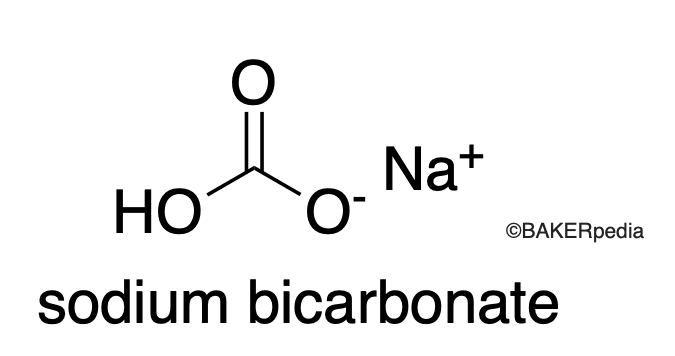
Chemical Structure
Origin
Sodium bicarbonate is considered one of the oldest leavening agents used in baked goods. It was first produced in 1791 by the French chemist Nicolas Leblanc and was introduced to the US in the 1840s by John Dwight and Austin Church.¹,²
Today, baking soda is a widely used chemical leavening agent due to its accessibility and affordability.
Function
Baking soda offers several benefits to baked goods, such as:¹
- Leavening: gases formed from the breakdown of baking soda allow the batter to expand during baking.
- Tenderizing: produced gases expand the cell walls of baked goods, making them more tender.
- pH adjustment: baking soda increases the pH of baked goods, this may have an effect on color, flavor, crumb texture, and gluten strength.
- Texture improvement: creates a finer crumb.
- Adds flavor: in certain baked goods, it may provide a distinctive salty flavor.
Nutrition
Baking soda contains a high amount of sodium that may be detrimental for heart health.
Commercial production
Baking soda is commercially produced through the following process: 4
- Synthesis: sodium carbonate solution is treated with carbon dioxide to form a slurry/suspension.
- Filtering: the suspension is separated from the liquid, producing a cake.
- Washing: the cake is washed in a bicarbonate solution.
- Drying: drying in a belt conveyor or flash dryer.
- Particle size reduction: the dried powder is milled to desired particle size.
Other methods include:
- Reaction of carbon dioxide with an aqueous solution of sodium hydroxide.
- Solvay method, which is based on reacting sodium chloride, ammonia and carbon dioxide in water.
Application
Baking soda can be used in the manufacture of several baked goods like cookies, cakes, muffins and cupcakes. ¹,²,³
Some considerations when using baking soda:
- If used in excess, it may cause a yellow or green dislocation and a strong chemical flavor.
- Some acidic products like buttermilk, sour cream, vinegar or yogurt can be used with baking soda. However, the difference in acid content of each one must be considered.
- It must be added with dry ingredients to prevent immediate reaction.
- The finer the particle size, the more reactive the baking soda. Coarse granular baking soda must be used in dry cake mixes.
- For low sodium baking, it can be substituted with potassium bicarbonate.
Usage levels and impact of baking soda on select baked goods: ¹,²,³
| Baked goods | Usage level | Advantage | Disadvantage |
| Acid batters | 2-3% | Improves textural properties by neutralizing the acid | In excess:
|
| Brownies | 2-3% | In small amounts:
|
In excess:
|
| Gingerbread cookies | 2-3% | ||
| Cookies | 0.5-1% | In small amounts:
|
In excess:
Especially with buttermilk, it may develop a soapy flavor. |
| Soda-acid biscuits | 3% | More tenderness | |
| White bread | 3% | Similar taste and texture to yeast-leavened breads | Texture can be improved by adding ethanol. |
Regulations
Baking soda is considered GRAS by the FDA when used within current good manufacturing practices. It is regulated by the CFR Title 21 Part 184.4
In the EU, baking soda (E 500 ii) is regulated by the EU commission No 231/2012.5
References
- Figoni, P. How Baking Works: Exploring The Fundamentals Of Baking Science. 2nd ed., John Wiley & Sons, Inc., 2008.
- Edwards, W. P. The science of bakery products. Royal Society of chemistry, 2007.
- NIIR Board of Consultants & Engineers.The Complete Technology Book On Bakery Products. 3rd ed., NPCS, 2014.
- U.S. Department of Health and Human Services.” Direct Food Substances Affirmed As Generally Recognized As Safe”.Title 21 Code of Federal Regulation, Part. 184. April 2019. Available at https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=184.1736 . Accessed 17 September 2020.
- European Commission (EC). Commission Regulation NO231/2012 laying down specifications for food additives listed in Annexes II and III to Regulation (EC) No 1333/2008 of the European Parliament and of the Council . Official Journal of European Communities, 09 March 2012.